Supporting researchers in conducting clinical research within the U-M Health Statewide Network of Care.
University of Michigan Health (U-M Health), Michigan Medicine is committed to ensuring Michiganders have access to the right care in the right place, and therefore has developed several collaborations and affiliations with health systems, hospitals, and providers throughout the state so that patients can receive care closer to home.
Extending clinical research throughout this statewide network of care in order to provide more opportunities for these patients to participate in research to advance health that serves Michigan and the world is an integral part of the vision and requires significant coordination. The Medical School Office of Research and the Michigan Institute for Clinical & Health Research (MICHR) have developed a team to ensure that researchers across the U-M Health Statewide Network of Care are supported in extending clinical research opportunities to patients, while also adhering to the unique administrative and regulatory requirements that are needed when working across affiliated and collaborating sites.
More information about the U-M Health Statewide Network of Care
Clinical research with the U-M Health Statewide Network of Care is different from multi-site research because:
- In typical multi-site research, the collaborating institutions are entirely separate entities. The clinical collaborations and affiliation arrangements that have been established introduce additional considerations.
- In typical multi-site research, each institution has a site Principal Investigator who is affiliated only with the site and responsible for executing the study. In the collaborative clinical relationships within the U-M Health Statewide Network of Care, researchers with a primary affiliation with one U-M Health Network institution may conduct research in another U-M Health Network institution’s facility.
- Multi-site research commonly occurs at academic medical centers. Administrative infrastructures for research differ between academic medical centers and non-academic healthcare systems. In particular, at U-M, the academic medical center is part of, and relies upon the university’s administrative research infrastructure. Non-academic healthcare systems do not have a parallel system like that of a university.
- Although U-M researchers commonly conduct multi-site research with non-academic partners, the scale, volume, and complexity of this research is greater.
The nature of the institutional relationships within the U-M Health Statewide Network of Care introduces the need for specific research requirements that must be addressed before any study activities can begin.
The first step for U-M researchers who are interested in conducting research at U-M Health Statewide Network locations is to submit a U-M Health Statewide Network of Care Intake Form so that the Statewide Clinical Research Partnerships (SCRP) team will help with navigate the necessary regulatory and administrative processes and requirements that need to be addressed when working across U-M Network of Care sites.
Approvals for research conducted at U-M do not automatically extend to the U-M Health Statewide Network of Care sites. No research activities may occur at/with any U-M Health Statewide Network of Care sites until necessary approvals are obtained.
All U-M Health Statewide Network of Care sites are separate entities from U-M and must be treated as such. In particular, U-M Health West and U-M Sparrow are separate entities from U-M for the purposes of research. For example, a study approved by a U-M IRB has not necessarily been IRB-approved to be conducted at a U-M Health Statewide Network of Care site.
IRB
Adding study activities at/with a U-M Health Statewide Network of Care site will require changes to the study’s eResearch application. The details depend on whether the U-M Health Network of Care site will be engaged in the research activity and who will be providing IRB oversight (IRBMED, IRB-HSBS or an external IRB). Considerations are outlined in this practice guide.
External sponsor awards and contracts
Adding U-M researcher study activities at a U-M Health Statewide Network of Care site may require changes to the study’s award or contract (if externally funded). Changes may include adding performance site(s) to the award and/or changing the class code to reflect the off-campus indirect cost rate. Engaging a U-M Health Statewide Network of Care site as an independent performance site led by a non-U-M clinician will require subcontracts. SCRP will help study teams navigate when and which U-M offices to contact about this.
It is very important to identify the specific rooms within the buildings where the research will occur. Regulatory and administrative rules and processes vary depending on whether the square footage in which the research activity is taking place is:
- Wholly owned and operated by U-M (note: U-M Health West and U-M Sparrow are NOT considered to be wholly owned and operated by U-M for the purposes of research)
- Leased by U-M
- Wholly owned and operated by the affiliate partner.
It is the U-M study team’s responsibility to notify SCRP if/when there are study team personnel changes that involve research activities at/with U-M Health Network of Care sites. Study teams must notify the SCRP when a U-M study team member who has access to the U-M Health Network of Care site’s electronic health record (EHR) or facility is no longer active on the project (or if access is no longer needed), so that this access can be terminated.
It is the U-M study team’s responsibility to notify SCRP of protocol changes that involve activities at/with U-M Health Network of Care sites so that any regulatory or administrative implications can be addressed before the revised protocol is implemented at the affiliate site.
Accessing the EHR for Research
Approval to access the EHR for clinical care does not automatically extend to research. Specific data use agreements for research may need to be established.
Care Everywhere cannot be used for research purposes.
Care Everywhere (CE) is an Epic functionality that allows clinical care providers to share a patient's health care record across health care systems to facilitate continuity of care. Epic’s “Rules of the Road” guidelines state that users may only access CE to request and view patient information for the purpose of treatment and care coordination. Access for research is explicitly not allowed when the EHR data have not already been accessed for clinical care coordination. The Rules do not have any specifications or restrictions on how the data may be used after it has been retrieved for treatment or care coordination purposes. Information that was originally requested for the purpose of treatment or care coordination, once received, may be accessible to researchers.
For specific study questions regarding using CE in research, reach out to the MiChart Research Team here (requires Level 2 login).
The joint venture between U-M Health and Trinity Health allows physicians to work collaboratively to care for patients at Chelsea Hospital.
Through the joint venture, patients who previously would have received inpatient rehabilitation at Michigan Medicine Ann Arbor will now receive care at Trinity Health’s Chelsea Hospital. There will no longer be an adult inpatient Physical Medicine and Rehabilitation service located at Michigan Medicine Ann Arbor after August 2024.
To ensure that patients have the opportunity to participate in the breadth of research available at U-M, the SCRP team has developed the following information for researchers:
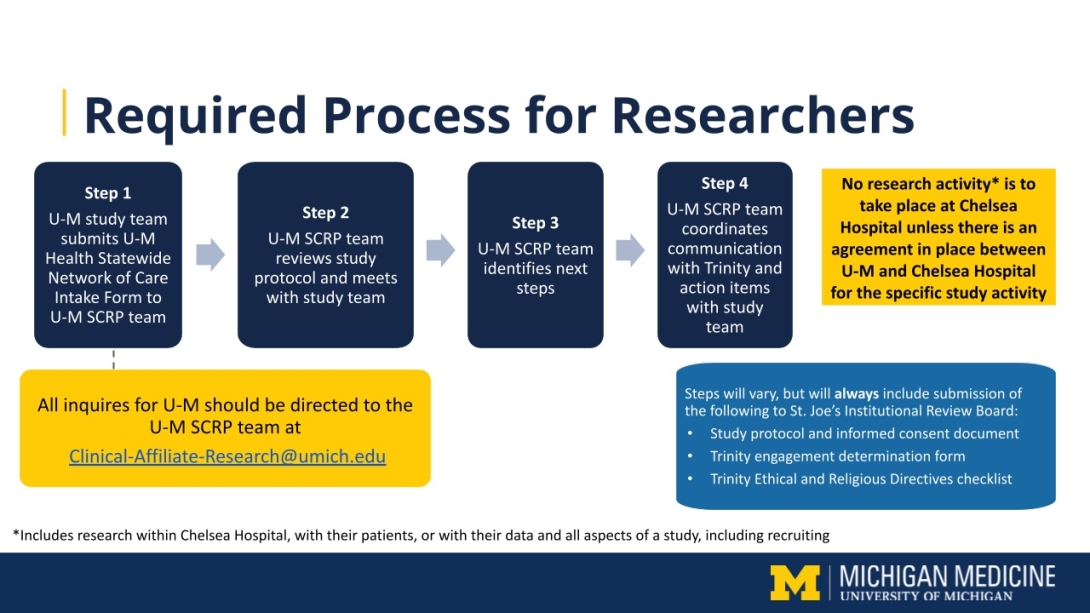
No research activity is to take place at Chelsea Hospital unless there is an agreement in place between U-M and Chelsea Hospital for the specific study activity (where activity includes research within Chelsea Hospital, with their patients, or with their data and all aspects of a study, including recruiting).
The process for getting started with U-M research at Chelsea Hospital is as follows:
Step 1: T U-M researchers who are interested in conducting research at Chelsea Hospital must submit an Intake Form to the SCRP team. Please know that if Chelsea Hospital receives an inquiry about starting a study, it will be directed to the SCRP at Clinical-Affiliate-Research@umich.edu.
Step 2: Once the form is received, the SCRP team will review it, as well as other study information.
Step 3: The SCRP team will meet with the study team.
Step 4: The SCRP team will provide the study team with next steps, as appropriate. These steps will vary but will always include submission of the following to ST. Joe’s Institutional Review Board:
- Study protocol and informed consent document
- Trinity engagement determination form
- Trinity Ethical and Religious Directives checklist
Note: U-M faculty, staff and trainees (i.e., postdocs, house officers, students) conducting research at Chelsea Hospital are acting as agents of U-M and therefore all U-M rules, regulations, policies and processes governing research apply.
Inpatients at Chelsea Hospital are Chelsea Hospital patients with Chelsea Hospital Medical Record Numbers (MRNs).
No. There are several steps that must be taken to ensure that the appropriate regulatory and administrative approvals are in place before any U-M study activity can begin involving patients at Chelsea Hospital. Please reach out to the SCRP team through the U-M Health Statewide Network of Care Intake Form or submit general inquiries through email (Clinical-Affiliate-Research@umich.edu) as early as possible before you intend to start your study. In addition, it is very important to reach out early in grant planning to address budgeting and contracting issues that are specific to research at or with Chelsea Hospital.
No, this is not an approved use of this function by Epic. You must establish the necessary approvals prior to accessing Chelsea Hospital EHR data for research.
No, this access is limited to view-only at this time. You may not enter orders in the Chelsea Hospital EHR. To be granted view-only EHR access, you will need to complete a series of steps.
No. Individuals requiring inpatient rehabilitation who are younger than age 18 years would receive care at C.S. Mott Children’s Hospital.