
The gift of research participation creates a better future
Degeneration of brain cholinergic pathways produces cognitive deficits which disrupt integration of cognitive, sensory, and motor functions critical for normal gait and balance.
The central theme of the University of Michigan Udall Center is the role of cholinergic deficits in treatment-refractory aspects of Parkinson disease (PD), particularly gait and balance disorders, and cognitive decline. Through an interrelated and complementary set of neuroimaging and behavioral studies in patients with PD and unique animal model experiments, Center investigators examine the contribution of distinct cholinergic projection system deficits to abnormalities of gait, balance, and cognition in PD. A major aspect of this central theme is the role of cholinergic systems in integrating afferent sensory, attentional, and cognitive information with motor functions.
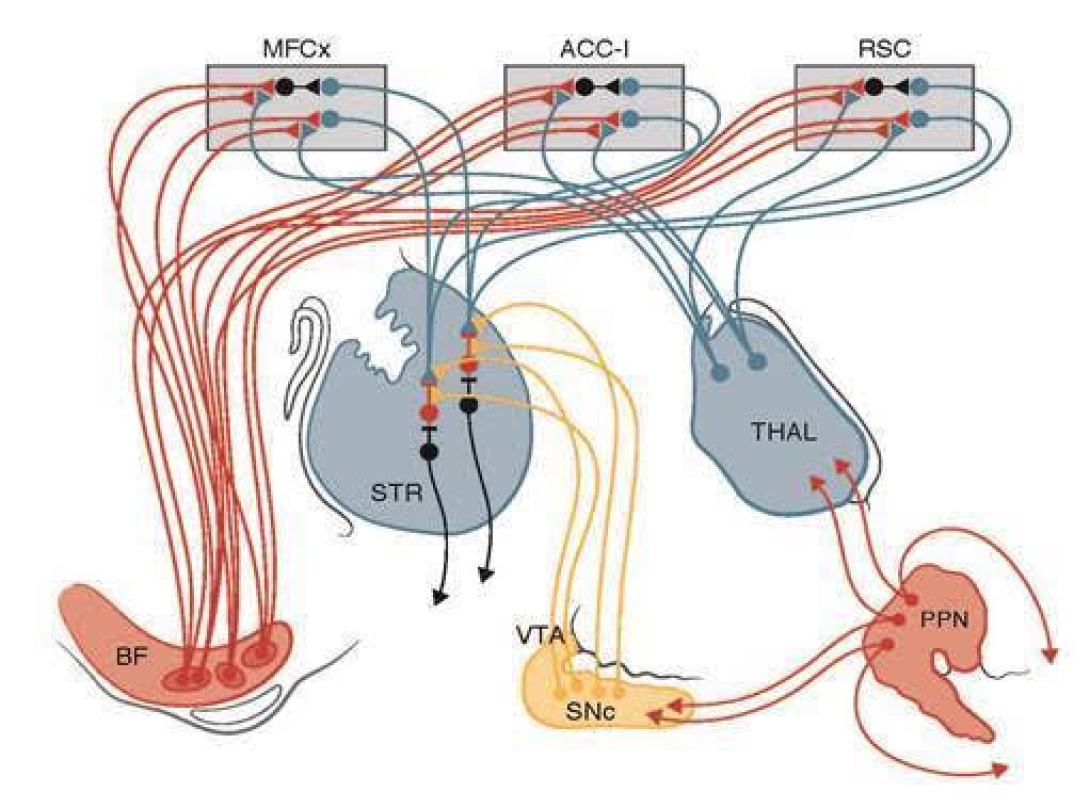
Attentional-Motor Interface
Multiple neurochemical systems degenerate in Parkinson’s Disease (PD). Progressive gait and balance difficulties, with associated falls, are among the most common levodopa resistant symptoms, eventually occurring in nearly all patients. The consequences of these levodopa resistant symptoms are devastating, and include bone fractures, hospitalizations, self-imposed isolation because of fear of falling, wheelchair confinement, and eventual nursing home placement. Similarly, cognitive decline is a prominent, common, and disabling feature of advancing PD, overlapping significantly with gait and balance disorders.
Data collected by Center investigators indicates that gait and postural control are not purely “motor” functions but require complex integration of motor, sensory, and cognitive functions. Defining the relationship between cholinergic dysfunction, gait abnormalities, and cognitive decline requires a multidisciplinary approach in which investigators view the relationship between cholinergic function, gait, and cognition through different lenses, share insights and challenge each other in ways that yield progress far beyond that achievable were each project pursued separately. The research team at the Center developed data that led to the development of a conceptual model of cognitive-motor integration (the Attentional-Motor Interface above) in which cholinergic systems’ interactions with basal ganglia and cortical circuits are critical for normal function. This model provides a systems-level framework for understanding the interactive nature of gait, balance, and cognitive dysfunctions in PD and focuses experimental attention on critical nodes of this system.
The lack of effective therapies for gait, balance, and cognitive abnormalities in advancing PD stems in large part from a limited understanding of the role of non-dopaminergic systems in the pathophysiology of these symptoms. It is increasingly clear that normal gait and balance depends upon complex interplay of motor, sensory and cognitive functions, indicating that the full spectrum of PD neuropathology must be considered to identify the responsible neural substrates. The molecular imaging work of Udall Center investigators demonstrated that PD subjects with a history of falls and/or gait freezing have differing deficits of specific cholinergic systems. Parallel work with a unique animal model demonstrated the critical role of cholinergic systems subserving attentional functions in normal gait and balance functions. In recent preliminary work, Udall Center molecular imaging data shows that other cholinergic deficits are related to other important aspects of motor and cognitive functions. Complementary experimental animal model work is illuminating the specific cellular mechanisms by which the cholinergic systems and nodes identified as abnormal by Udall Center clinical research integrate diverse circuits to produce normal cognitive and motor functions. All of this work is a necessary prelude to developing interventions for these treatment refractory aspects of PD.
Project Lead: Nicolaas Bohnen, MD, PhD
Postural instability and gait difficulty (PIGD) motor features are common in Parkinson disease (PD), and a significant cause of treatment-refractory disability. Accumulating evidence implicates cholinergic systems dysfunctions as significant contributors to gait and balance impairment. During the initial funding period, we established the vesicular acetylcholine transporter (VAChT) ligand [18F]FEOBV, which uniquely assesses cholinergic terminal density in high density regions such as the striatum. Our recent cross-sectional findings suggest that PwP participants with isolated falls and those with freezing of gait (FoG) status share common cholinergic deficits in the thalamus (lateral geniculate nucleus [LGN]) and striatum (caudate) with more extensive striatal, limbic, and prefrontal VAChT reductions in PwP with FoG. Consistent with Project II preclinical data indicating a critical role for striatal cholinergic interneurons (SChI) in integration of attentional and motor functions, these data suggest that SChI deficits are a common denominator in the etiology of falls and FoG. These results emphasize the need to understand PIGD, falls, and FoG as products of cholinergic projection dysfunctions within the framework of failing Attentional-Motor Integration (AMI) combined with failures of additional multisensory and cognitive integration.
Episodic mobility disturbances (falls, FoG) are typically preceded by insidiously developing non-episodic PIGD features. We have novel preliminary data that cholinergic deficits of the medial geniculate nucleus (MGN) and the entorhinal cortex (ERC) are robustly associated with non-episodic PIGD deficits, These results imply a significant role of impaired sensorimotor integration underlying non-episodic PIGD motor features in PwP. The overarching goal of this project is to investigate the evolution of cholinergic deficits within multisensory, cognitive and motor integration brain regions and development of PIGD features in PwP. We hypothesize that this progresses from the MGN and ERC, then LGN and caudate nucleus, and then more diffuse striatal, limbic and cortical (prefrontal followed by anterior cingulum and insula) cholinergic deficits. To assess our hypotheses, we propose to perform a prospective cohort study with [18F]FEOBV brain PET at baseline and 2-year follow-up in PD subjects at risk of conversion to non-episodic and episodic (falls and FoG) PIGD motor features. Novel insights in cholinergic changes underlying incident development of PIGD may inform new therapeutic interventions to treat these debilitating motor complications. Project I is highly integrated thematically with Project II and the Catalyst Research Project, complementary to Project III, and will interact extensively with all Cores. Our work is based on a unique, deeply phenotyped cohort of PD participants developed in the prior funding cycle allowing us to recruit an enriched sample of patients likely to convert to fall and FoG status, allowing longitudinal within-subject assessments.
[18F]FEOBV PET delineates specific cortical regions and deep brain structures, including striatum and thalamic subnuclei. PD subjects with falls vs those with falls and gait freezing exhibit distinct abnormal patterns, highlighted by reduced striatal [18F]FEOBV as a shared dysfunctional node. Dr. Bohnen and his team will prospectively test the hypothesis that the cholinergic AMI network dysfunctions they describe are core features of PD gait and balance dysfunction, and that distinct patterns of cholinergic pathology predict specific features of PD gait dysfunction. The serial assessments proposed will allow unique within-subject analysis for the temporal dissection of distinct and converging elements of mobility control deficits of gait-balance motor features in PD. Unlike the prior grant cycle where longitudinal assessment was limited, esp. for PwP converting from non-fallers/freezers to fallers/freezers, the current cycle will allow clinically meaningful follow-up assessments of up to 5-6 years of PwP who completed baseline [18F]FEOBV PET. Hypotheses to test: 1) whether incident fallers exhibit [18F]FEOBV defects in the caudate, visual thalamus, and prefrontal cortex (compared to non-converters), and 2) whether the subsequent emergence of gait freezing involves additional and more widespread cholinergic vulnerability of the striatum, limbic archicortex, including the cingulo-opercular and insular cortices. The existence of the unique PD subject cohort developed in the currently funded cycle, together with newly recruited subjects, will allow study of an enriched sample of those converting to falls and/or to gait freezing. The focus on visual thalamus, based on the repeated association of falls with cholinergic dysfunction (found with both [11C]PMP and [18F]FEOBV), is another important and novel element. Many thalamic nuclei are primarily interconnected with association cortices with these thalamic nuclei are increasingly viewed as partners and/or modulators of cortical functions. Emerging evidence supports critical roles of visual thalamus in mediating visual attention. LGN function is modulated by attention, indicating a key role for this relay structure in bottom-up attention. Dr. Bohnen has new data implicating cholinergic denervation of additional regions driving non-episodic PIGD deficits preceding falls and FoG. This data indicates that cholinergic deficits within the MGN and entorhinal cortex (EC) are robustly associated with non-episodic PIGD, independent of nigrostriatal dopaminergic deficits. MGN is involved in processing multi-sensory (auditory, vestibular and proprioceptive) inputs, implying a significant role of impaired sensorimotor integration underlying early PIGD features in PwP. EC is associated with visuospatial maps, suggesting deficient attention-visuomotor integration. Complementary work will be performed in the Catalyst Research Project. The analysis of cholinergic system changes of the evolution of balance and gait disturbances will be complemented by exploratory mechanistic multisensory and attentional-motor integration studies. These studies will identify the key patterns of cholinergic systems dysfunction underlying treatment-refractory gait and balance disorders in PwP.
Project Lead: Kent Berridge, PhD (replacing Martin Sarter, PhD)
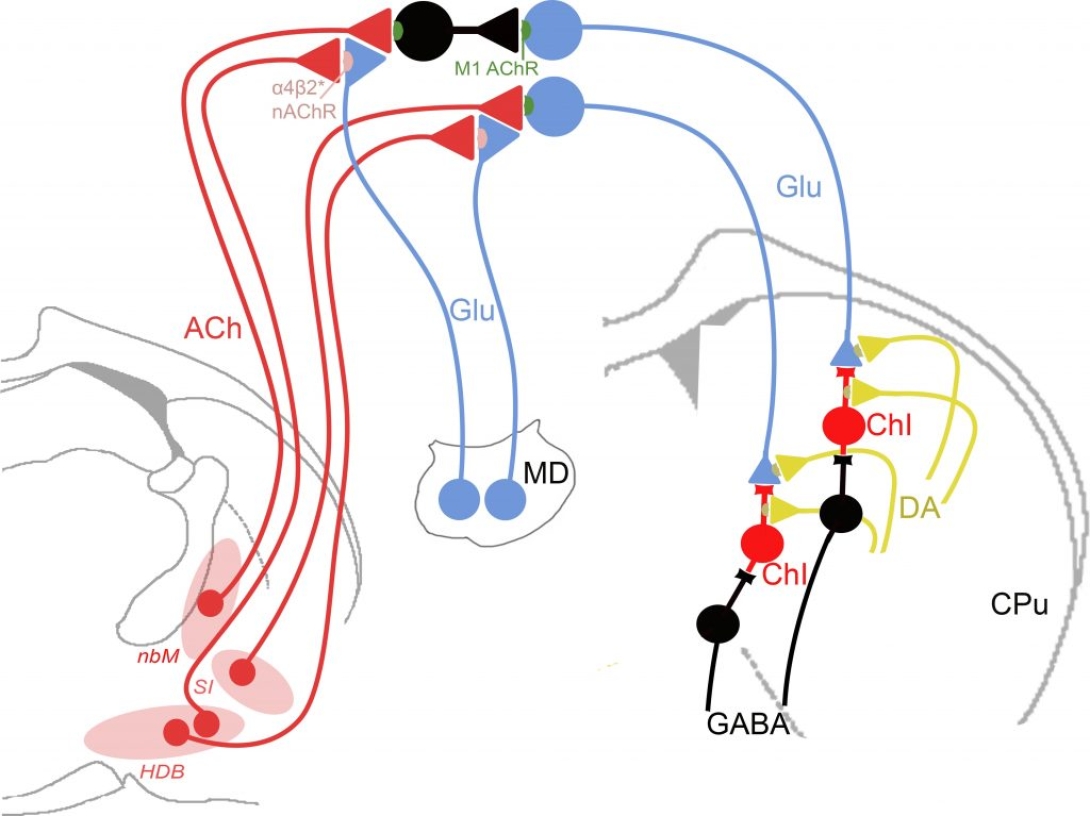
This project builds on Dr. Martin Sarter’s published and preliminary work dissecting the mechanisms of the detection of relevant cues, transfer of this information to striatum, and its integration with ongoing motor processing. Based on the Sarter team’s demonstration of a role for basal forebrain cholinergic projections in the DL (dual lesion) rodent model of PD falling, this project uses optogenetic techniques to test the hypothesis that an enhanced fall propensity can be caused specifically by the loss of fast cholinergic signaling (i.e., “transients”) known to mediate attention. Additional experiments define how cholinergic-mediated cortical attentional information is integrated with motor selection processes. Frontostriatal circuitry (glutamatergic corticostriatal projections) is essential for action planning, particularly when habit-guided action is disrupted and task shifts are needed. Such adjustment depends critically on corticostriatal information transfer to guide adaptive movement selection and sequencing. Deficient cortical cholinergic activity impairs attentional shifts toward alternative actions, uncoupling striatal action selection from goals, causing ill-timed or absent responses. Guided by this framework, intrinsic to the AMI model, Dr. Sarter’s team acquired compelling preliminary data detecting the signals encoding attentional information transferred to the striatum (via corticostriatal terminals), where it is hypothesized to be integrated with vigor and kinematic signals supplied by nigrostriatal dopaminergic terminals. Utilizing a novel behavioral paradigm, they demonstrate that a cue instructing the animal to turn evokes a time-locked increase of striatal glutamate (that will be shown to originate from corticostriatal afferents), and a similarly timed burst of acetylcholine from cholinergic neurons. Dr. Sarter’s former team, now under the direction of Dr. Berridge, is specifically testing the hypothesis that this integrative function is essential for complex motor control, including PD gait dysfunction. Preliminary findings strongly support a role for cholinergic signals in integrating the attentional and motor signals during gait; chemogenetic activation of these neurons reduces fall propensity in DL rats (often preceded by freezing-like motor behaviors in the animals), whereas their inhibition in intact animals mimics DL-type falls. These data support key aspects of the AMI model by implicating cholinergic neurotransmission at two successive nodes along the cortico-striatal pathway critical to support the attentional-motor interface – in cortex for signal detection, and in striatum for signal integration. These studies will identify key substrates of attentional-motor integration in the AMI.
Project Lead: Roger Albin, MD
Cognitive deficits are a morbid dopamine replacement therapy-refractory feature of Parkinson disease (PD). The pathophysiology of PD-related cognitive deficits is complex, likely involving interacting and variable impairments of several brain systems, particularly in early to moderate disease. Incidence and natural history of PD cognitive deficits is heterogeneous. Understanding the pathophysiologies of PD cognitive impairments is essential for development of personalized therapies. PD heterogeneity is a major obstacle to effective clinical research. Identifying PD subgroups will enhance discovery of useful interventions through subgroup specific or stratified clinical trials, identify biomarkers, improve prognosis assessment in clinical care, and assist etiopathogenic research. Some of the “highest priority recommendations” of the NINDS PD 2014 Research Report call for research to understand the pathophysiology of cognitive impairments and for PD subgroup identification. The U-M Udall Center established a deeply phenotyped PD cohort imaged with the vesicular acetylcholine transporter PET ligand [18F]FEOBV, revealing heterogeneous cholinergic deficits. Cholinergic terminal deficits in Cingulo-Opercular Task Control network (COTC) nodes – Anterior Cingulate and Insular Cortices (AC-I) – correlate with both domain specific and global cognitive deficits. An important component of the Attentional-Motor Interface, the COTC subserves tonic attention, coordinating network activities across different cognitive domains. Preliminary analysis suggests that early COTC node (AC-I) cholinergic deficits are a subgroup defining-feature in PD, predicting more rapid cognitive decline. The central hypothesis of Project III is that early COTC node (AC-I) cholinergic denervation contributes significantly to cognitive impairment in early to moderate PD and identifies a D subgroup with accelerated cognitive decline In addition to our established Udall subject cohort, we have access to a separate cohort of incident PD subjects through collaboration with the University of Groningen, deeply phenotyped and imaged with [18F]FEOBV PET, for rigorous experimental replication and validation of our primary hypothesis. We will correlate early COTC node (AC-I) cholinergic denervation with domain-specific and general measures of cognitive function. In a prospective analyses, we will determine if early COTC node (AC-I) cholinergic denervation predicts more rapid cognitive decline. In an integrated analysis with Project I, we will determine if COTC node (AC-I) cholinergic denervation is associated with Freezing of Gait (FoG). In exploratory analyses, we will assess if more accessible MRI or other measures correlate with COTC node (AC-I) cholinergic denervation, identifying potential, accessible biomarkers of COTC node (AC-I) cholinergic denervation. Project III will identify an important substrate of PD cognitive impairment and identify a PD subgroup with a more aggressive natural history – a “malignant hypocholinergic disease phenotype.” These results will identify potential targets for therapeutic interventions and biomarker development.
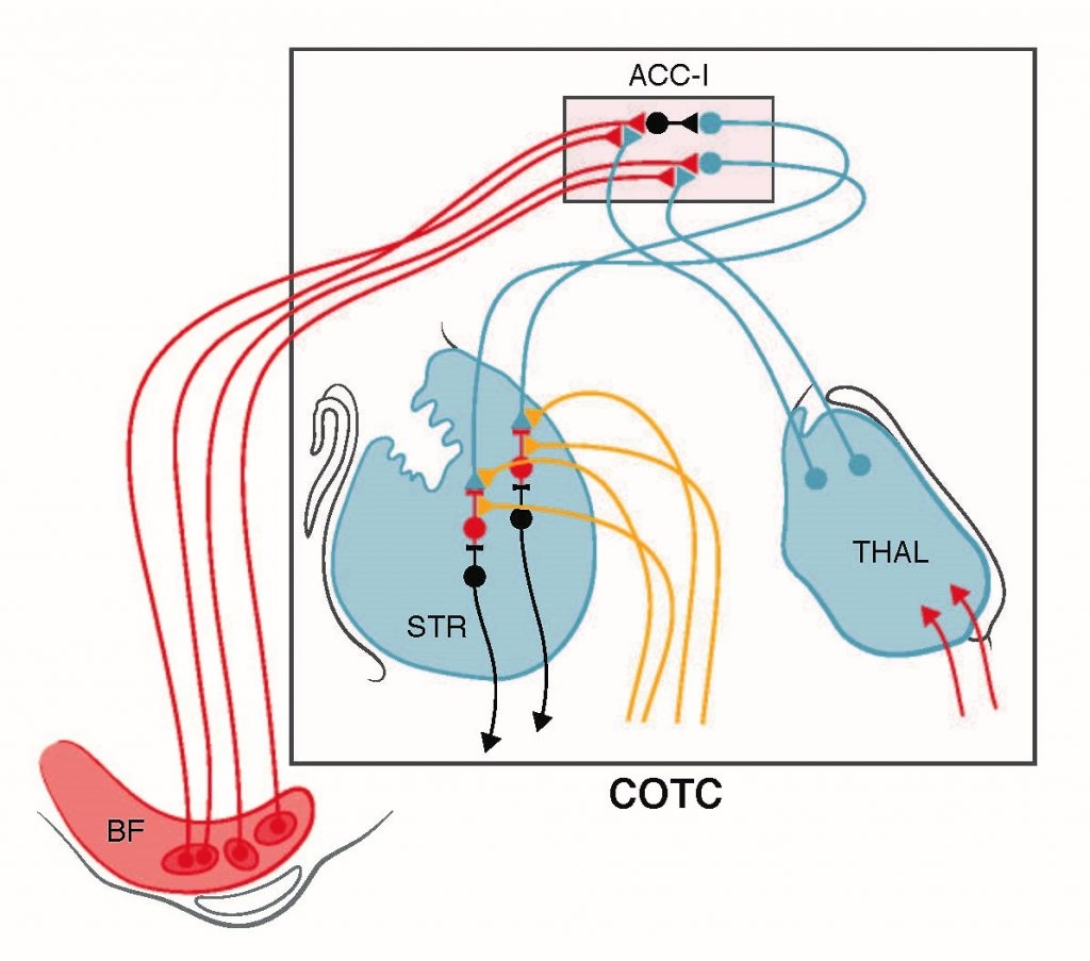
Our preliminary results indicate a global effect of key AMI node AC-I cholinergic denervation on cognitive functions. These cortical regions are key nodes of the COTC network and participate in higher level aspects of attentional function. We will confirm that AC-I denervation is associated with widespread cognitive deficits. Our established PD cohort, extensively characterized with dopaminergic and cholinergic PET, and with motor and cognitive assays, uniquely positions us to define the distinctive natural history of a PD subgroup based on a pathologic marker. Continued follow-up of this valuable cohort will assess if AC-I cholinergic denervation predicts significantly greater global cognitive decline. Quantification of cholinergic changes in the COTC subcortical and cortical (AC-I) network in this cohort will also allow the assessment that progressive cognitive changes coincide with more severe motor changes (aggressive or ‘malignant’ PD subtype). PET studies are costly and [18F]FEOBV is only operational in a handful of centers. A convenient, reliable predictor of AC-I cholinergic denervation is required to employ this phenotype as subgroup marker or stratifying method. We will capitalize on the thorough phenotyping of our cohort and apply more convenient MRI methods, complementing Project I analyses, to explore an accessible biomarker. Through collaboration with investigators at the University of Groningen (Netherlands), we have access to a similarly characterized and prospectively followed cohort of incident PD subjects who also undergo [18F]FEOBV PET. These subjects are part of the Dutch Parkinson and Cognition Study (DUPARC). This collaboration will enable us to rigorously test our hypotheses regarding the impacts and prognostic potential of AC-I cholinergic denervation and identification of a useful predictor in an independent replication cohort. Our Groningen colleagues agreed to share all phenotypic, MRI, and [18F]FEOBV imaging data to address, in parallel, the hypotheses driving Project III. These studies will identify a key substrate of cognitive impairments and predictor of more rapid cognitive decline in PwP.
Project Lead: Omar Ahmed, PhD
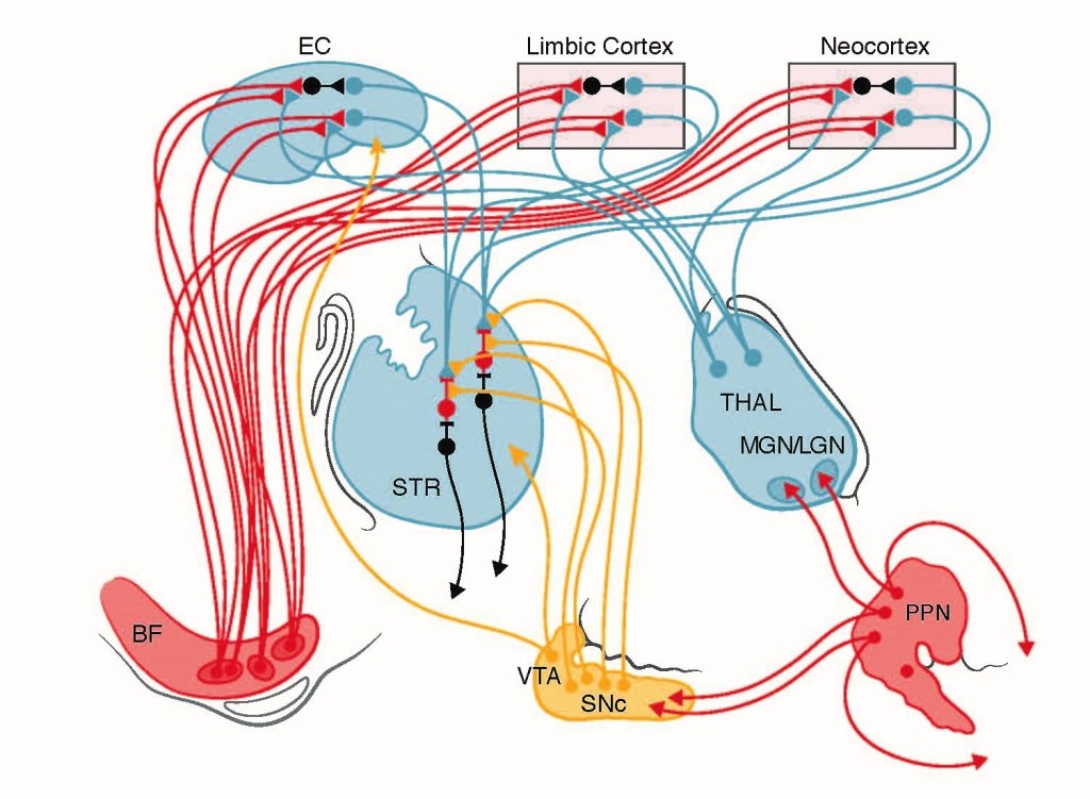
Many patients with Parkinson’s disease (PD) suffer from spatial disorientation – inability to link external landmark cues to internal estimates of self-orientation. These deficits are not improved by dopamine replacement therapy (DRT). The same spatial disorientation features are found in patients with specific lesions, due to a stroke or hemorrhage, of the retrosplenial cortex (RSC), a brain region critical for encoding the combination of allocentric and egocentric navigational information. Attentional and emotional processing impairments in PD patients are accompanied by altered BOLD responses in the retrosplenial cortex. The retrosplenial cortex is densely interconnected with the secondary motor cortex, hippocampus, visual cortex, cingulate cortex and anterior thalamus (containing head orientation cells), and is therefore part of the Attentional-Motor Interface (AMI) and ideally positioned to help transform attentional and spatial information into planned actions. Furthermore, multiple basal forebrain structures send cholinergic projections to the RSC. There are pronounced increases in acetylcholine (ACh) release in the retrosplenial cortex during attentive spatial navigation. Cholinergic deficits, such as those seen in PD, are likely to severely impair the spatial orientation functions of the retrosplenial cortex. Little is known about 1) how cholinergic inputs influence the synapses, cells and circuits of the retrosplenial circuits, and 2) the impact of cholinergic dysfunction on retrosplenial-dependent spatial orientation and navigation. Our central hypothesis is that dysfunctional cholinergic systems projecting to the retrosplenial cortex will manifest in altered navigational encoding by retrosplenial circuits and spatially disoriented behaviors. We will decipher the mechanisms of cholinergic control of retrosplenial cells and synapses, with preliminary data suggesting both cell-type- and synapse-specific cholinergic controls. We will investigate how the loss of cholinergic inputs impairs retrosplenial encoding of space and how it impacts orientation-guided movement. These investigations will elucidate the contributions of the retrosplenial orientation coding circuit to the Attentional-Motor Interface, and lay the groundwork for understanding how altered perception of spatial orientation in Parkinson’s disease can directly impact motor control.
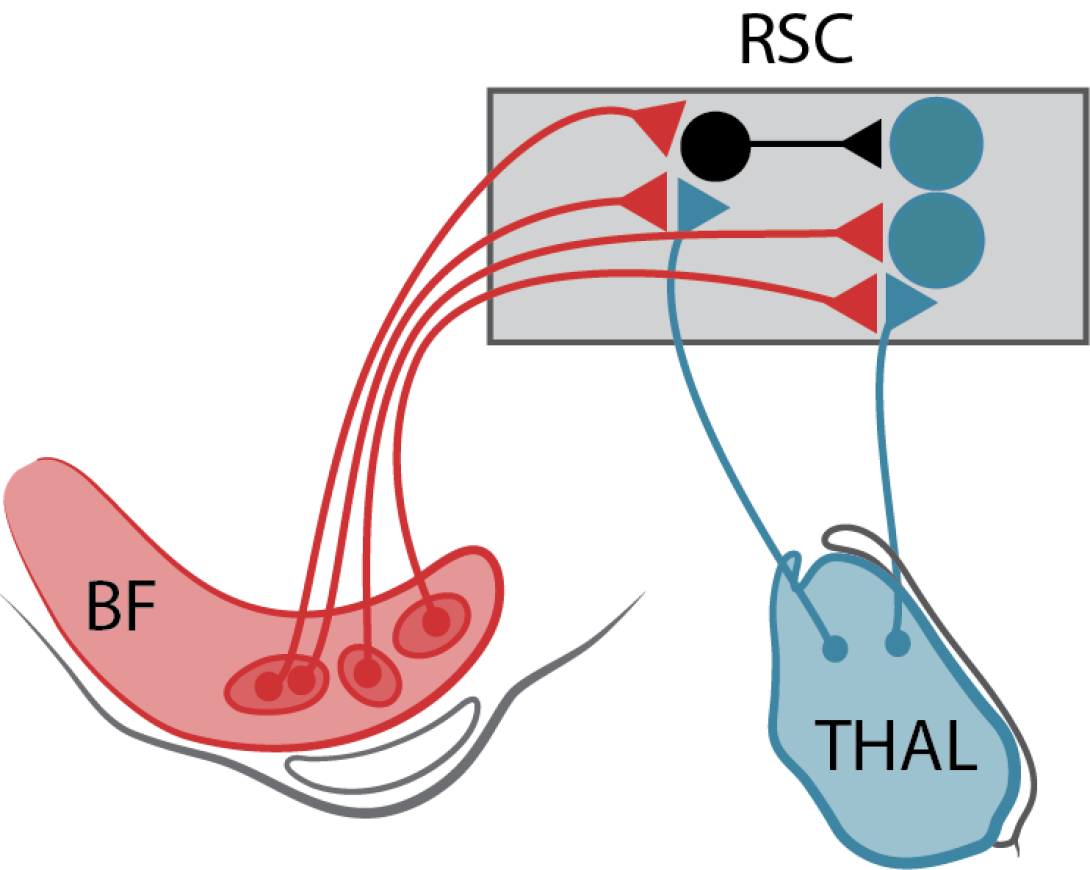
Many PwP experience DRT-refractory spatial disorientation with inability to integrate external landmark cues with internal estimates of orientation. The retrosplenial cortex (RSC) is critical for this function with fMRI studies indicating abnormal attentional information processing in PwP. RSC, an integral AMI component, is densely interconnected with other AMI nodes, the cingulate cortex and anterior thalamus (locus of head position neurons), and receives dense BF cholinergic afferents. These cholinergic inputs are critical for attentive spatial navigation. In work funded by a U-M Udall Center Pilot Project, Dr. Ahmed uncovered a unique pattern of local inhibition in the RSC. In this Catalyst Research Project, Dr. Ahmed will evaluate the hypotheses that thalamic input mediated spatial information is modulated by BF cholinergic afferents and that cholinergic receptor mediated responses in RSC neurons are critical for maintaining attentive navigation. RSC inter-hemispheric communication is necessary to maintain attentive navigation. Dr. Ahmed specifically hypotheses that cholinergic signaling is necessary for normal inter-hemispheric RSC function. Dr. Ahmed will evaluate these hypotheses in both normal animals and in the dual lesion (DL) model of combined striatal dopaminergic denervation and cortical cholinergic denervation developed by Dr. Sarter. This study will identify mechanisms of information transfer within a key AMI node. These experiments provide a cellular level examination of cholinergic functions within an AMI node, complementing the systems and circuit level approaches of the other projects. The focus on attentive spatial navigation also complements Project I studies assessing the roles of deficient sensorimotor and visuomotor integration in PwP.
- Discussion of Research Priorities for Gait Disorders in Parkinson's Disease. Bohnen NI, Costa RM, Dauer WT, Factor SA, Giladi N, Hallett M, Lewis SJG, Nieuwboer A, Nutt JG, Takakusaki K, Kang UJ, Przedborski S, Papa SM; MDS-Scientific Issues Committee. Mov Disord. 2022 Feb;37(2):253-263. doi: 10.1002/mds.28883. Epub 2021 Dec 22. PMID: 34939221; PMCID: PMC10122497.
- Striatal Dopamine: The Cement of the Brain?: Jeong H, Taylor A, Floeder JR, et al. Mesolimbic dopamine release conveys causal associations. Science 2022;378:eabq6740.: Jeong H, Taylor A, Floeder JR, et al. Mesolimbic dopamine release conveys causal associations. Albin RL, Leventhal DK. Science 2022;378:eabq6740. Mov Disord. 2023 May;38(5):742. doi: 10.1002/mds.29379. Epub 2023 Mar 31. PMID: 37002590. PMCID: PMC10790133
- Brain Dynamics Underlying Preserved Cycling Ability in Patients With Parkinson's Disease and Freezing of Gait. Licen T, Rakusa M, Bohnen NI, Manganotti P, Marusic U. Front Psychol. 2022 Jun 16;13:847703. doi: 10.3389/fpsyg.2022.847703. PMID: 35783714; PMCID: PMC9244145.
- Emerging non-invasive therapeutic approaches targeting hypocholinergic neural systems in Parkinson's disease. Bohnen NI, Yarnall AJ. Neural Regen Res. 2023 Apr;18(4):809-810. doi: 10.4103/1673-5374.353490. PMID: 36204846; PMCID: PMC9700120.
- Multimodal gradients of human basal forebrain connectivity. Chakraborty S, Haast RAM, Kanel P, Khan AR, Schmitz TW. bioRxiv [Preprint]. 2023 May 26:2023.05.26.541324. doi: 10.1101/2023.05.26.541324. PMID: 37292595; PMCID: PMC10245994.
- Cholinergic system correlates of postural control changes in Parkinson's disease freezers. Roytman S, Paalanen R, Griggs A, David S, Pongmala C, Koeppe RA, Scott PJH, Marusic U, Kanel P, Bohnen NI. Brain. 2023 Aug 1;146(8):3243-3257. doi: 10.1093/brain/awad134. PMID: 37086478; PMCID: PMC10393403.
- Cholinergic centro-cingulate network in Parkinson disease and normal aging. Bohnen NI, van der Zee S, Albin R. Aging (Albany NY). 2023 Oct 27;15(20):10817-10820. doi: 10.18632/aging.205209. Epub 2023 Oct 27. PMID: 37899134; PMCID: PMC10637805.
- Regional serotonin terminal density in aging human brain: A [11C]DASB PET study. Kanel P, Koeppe RA, Kotagal V, Roytman S, Muller MLTM, Bohnen NI, Albin RL. Aging Brain. 2023 Mar 1;3:100071. doi: 10.1016/j.nbas.2023.100071. PMID: 37408789; PMCID: PMC10318302.
- Challenges and innovations in brain PET analysis of neurodegenerative disorders: a mini-review on partial volume effects, small brain region studies, and reference region selection. Kanel P, Carli G, Vangel R, Roytman S, Bohnen NI. Front Neurosci. 2023 Nov 30;17:1293847. doi: 10.3389/fnins.2023.1293847. PMID: 38099203; PMCID: PMC10720329.
- A Positron Emission Tomography Ligand for Mutant Huntingtin Sheds Light on Disease. Lieberman AP, Albin RL. Mov Disord. 2022 May;37(5):893. doi: 10.1002/mds.29019. Epub 2022 Apr 9. PMID: 35396866; PMCID: PMC9182207.
- Neuroimaging statistical approaches for determining neural correlates of Alzheimer’s disease via positron emission tomography imaging. Drake DF, Derado G, Zhang L, Bowman FD. WIREs Computational Statistics (2023) DOI: 10.1002/2ics.1606
- Update: Descriptive epidemiology of Parkinson disease. Grotewold N, Albin RL. Parkinsonism Relat Disord. 2024 Jan 8:106000. doi: 10.1016/j.parkreldis.2024.106000. Epub ahead of print. PMID: 38233324; PMC10922566.
- What is the Parkinson Pandemic? Albin R, Grotewold N. Mov Disord. 2023 Dec;38(12):2141-2144. doi: 10.1002/mds.29637. Epub 2023 Oct 20. PMID: 37859586; PMC10843050.
- Basal forebrain integrity, cholinergic innervation and cognition in idiopathic Parkinson's disease. Crowley SJ, Kanel P, Roytman S, Bohnen NI, Hampstead BM. Brain. 2023 Dec 18:awad420. doi: 10.1093/brain/awad420. Epub ahead of print. PMID: 38109781. PMCID: PMD11068112.
- Cholinergic innervation topography in GBA-associated de novo Parkinson's disease patients. Slingerland S, van der Zee S, Carli G, Slomp AC, Boertien JM, d'Angremont E, Bohnen NI, Albin RL, van Laar T. Brain. 2023 Sep 25:awad323. doi: 10.1093/brain/awad323. Epub ahead of print. PMID: 37748026; PMCID: PMC10907081
- Identification of cholinergic centro-cingulate topography as main contributor to cognitive functioning in Parkinson's disease: Results from a data-driven approach. van der Zee S, Kanel P, Müller MLTM, van Laar T, Bohnen NI. Front Aging Neurosci. 2022 Oct 20;14:1006567. doi: 10.3389/fnagi.2022.1006567. PMID: 36337707; PMCID: PMC9631831.
- Progression of regional cortical cholinergic denervation in Parkinson's disease. Bohnen NI, Roytman S, Kanel P, Müller MLTM, Scott PJH, Frey KA, Albin RL, Koeppe RA. Brain Commun. 2022 Dec 2;4(6):fcac320. doi: 10.1093/braincomms/fcac320. PMID: 36569603; PMCID: PMC9772878.
- Vestibular Sensory Conflict During Postural Control, Freezing of Gait, and Falls in Parkinson's Disease. Bohnen NI, Kanel P, van Emde Boas M, Roytman S, Kerber KA. Mov Disord. 2022 Nov;37(11):2257-2262. doi: 10.1002/mds.29189. Epub 2022 Aug 15. PMID: 36373942; PMCID: PMC9673158.
- Serotonin Transporter Imaging in Multiple System Atrophy and Parkinson's Disease. Chou KL, Dayalu P, Koeppe RA, Gilman S, Spears CC, Albin RL, Kotagal V. Mov Disord. 2022 Nov;37(11):2301-2307. doi: 10.1002/mds.29220. Epub 2022 Sep 14. PMID: 36102173; PMCID: PMC9669145.
- Dopamine neuron stimulation induces context-dependent abnormal involuntary movements in healthy rats. Hunter J, Bova A, Stevens A, Leventhal DK. iScience. 2022 Feb 22;25(3):103974. doi: 10.1016/j.isci.2022.103974. PMID: 35281727; PMCID: PMC8914546.
- Composite measures of motor performance and self-efficacy are better determinants of postural instability and gait difficulties than individual clinical measures in Parkinson's disease. Pongmala C, Roytman S, van Emde Boas M, Dickinson O, Kanel P, Bohnen NI. Parkinsonism Relat Disord. 2023 Feb;107:105251. doi: 10.1016/j.parkreldis.2022.105251. Epub 2022 Dec 22. PMID: 36566525; PMCID: PMC10028594.
- Automated production of [11C]butyrate for keto body PET imaging. Pakula RJ, Raffel DM, Koeppe RA, Winton WP, Stauff J, Bohnen NI, Albin RL, Scott PJH, Shao X. Nucl Med Biol. 2023 Jan-Feb;116-117:108315. doi: 10.1016/j.nucmedbio.2023.108315. Epub 2023 Jan 13. PMID: 36680983; PMCID: PMC10053181.
- Differential cholinergic systems' changes in progressive supranuclear palsy versus Parkinson's disease: an exploratory analysis. Kanel P, Spears CC, Roytman S, Koeppe RA, Frey KA, Scott PJH, Albin RL, Bohnen NI. J Neural Transm (Vienna). 2022 Dec;129(12):1469-1479. doi: 10.1007/s00702-022-02547-9. Epub 2022 Oct 12. PMID: 36222971; PMCID: PMC10017092.
- Cholinergic brain network deficits associated with vestibular sensory conflict deficits in Parkinson's disease: correlation with postural and gait deficits. Bohnen NI, Kanel P, Roytman S, Scott PJH, Koeppe RA, Albin RL, Kerber KA, Müller MLTM. J Neural Transm (Vienna). 2022 Aug;129(8):1001-1009. doi: 10.1007/s00702-022-02523-3. Epub 2022 Jun 26. PMID: 35753016; PMCID: PMC9308723.
- Atrophy of the Cholinergic Basal Forebrain can Detect Presynaptic Cholinergic Loss in Parkinson's Disease. Ray NJ, Kanel P, Bohnen NI. Ann Neurol. 2023 May;93(5):991-998. doi: 10.1002/ana.26596. Epub 2023 Jan 24. PMID: 36597786; PMCID: PMC10192078.
- Ketogenic interventions in mild cognitive impairment, Alzheimer's disease, and Parkinson's disease: A systematic review and critical appraisal. Bohnen JLB, Albin RL, Bohnen NI. Front Neurol. 2023 Feb 9;14:1123290. doi: 10.3389/fneur.2023.1123290. PMID: 36846143; PMCID: PMC9947355.
- Structural and molecular cholinergic imaging markers of cognitive decline in Parkinson's disease. Schumacher J, Kanel P, Dyrba M, Storch A, Bohnen NI, Teipel S, Grothe MJ. Brain. 2023 Dec 1;146(12):4964-4973. doi: 10.1093/brain/awad226. PMID: 37403733; PMCID: PMC10689921.
Automated production of [11C]butyrate for keto body PET imaging. Pakula RJ, Raffel DM, Koeppe RA, Winton WP, Stauff J, Bohnen NI, Albin RL, Scott PJH, Shao X. Nuclear medicine and biology. 2023 January;116-117:108315. PubMed PMID: 36680983; DOI: 10.1016/j.nucmedbio.2023.108315. PMCID: PMC10053181.
Atrophy of the Cholinergic Basal Forebrain can Detect Presynaptic Cholinergic Loss in Parkinson's Disease. Ray NJ, Kanel P, Bohnen NI. Ann Neurol. 2023 Jan 4. doi: 10.1002/ana.26596. Epub ahead of print. PMID: 36597786. PMCID: PMC10192078
- Cholinergic brain network deficits associated with vestibular sensory conflict deficits in Parkinson's disease: correlation with postural and gait deficits. Bohnen NI, Kanel P, Roytman S, Scott PJH, Koeppe RA, Albin RL, Kerber KA, Müller MLTM. Journal of neural transmission (Vienna, Austria : 1996). 2022 August;129(8):1001-1009. PubMed PMID: 35753016; PubMed Central PMCID: PMC9308723; DOI: 10.1007/s00702-022-02523-3.
- Decreased vestibular efficacy contributes to abnormal balance in Parkinson's disease. Bohnen NI, Roytman S, Griggs A, David SM, Beaulieu ML, Müller MLTM. Journal of the neurological sciences. 2022 September 15;440:120357. PubMed PMID: 35932698; PubMed Central PMCID: PMC9444904; DOI: 10.1016/j.jns.2022.120357.
- Progression of regional cortical cholinergic denervation in Parkinson's disease. Bohnen NI, Roytman S, Kanel P, Müller MLTM, Scott PJH, Frey KA, Albin RL, Koeppe RA. Brain Commun. 2022 Dec 2;4(6):fcac320. doi: 10.1093/braincomms/fcac320. PMID: 36569603; PMCID: PMC9772878.
- Vestibular Sensory Conflict During Postural Control, Freezing of Gait, and Falls in Parkinson's Disease. Bohnen NI, Kanel P, van Emde Boas M, Roytman S, Kerber KA. Mov Disord. 2022 Nov;37(11):2257-2262. doi: 10.1002/mds.29189. Epub 2022 Aug 15. PMID: 36373942; PMCID: PMC9673158.
- Serotonin Transporter Imaging in Multiple System Atrophy and Parkinson's Disease. Chou KL, Dayalu P, Koeppe RA, Gilman S, Spears CC, Albin RL, Kotagal V. Mov Disord. 2022 Nov;37(11):2301-2307. doi: 10.1002/mds.29220. Epub 2022 Sep 14. PMID: 36102173; PMCID: PMC9669145.
- Running speed and REM sleep control two distinct modes of rapid interhemispheric communication. Ghosh M, Yang FC, Rice SP, Hetrick V, Gonzalez AL, Siu D, Brennan EKW, John TT, Ahrens AM, Ahmed OJ. Cell reports. 2022 July 5;40(1):111028. PubMed PMID: 35793619; PubMed Central PMCID: PMC9291430; DOI: 10.1016/j.celrep.2022.111028.
- Molecular Imaging of the Cholinergic System in Alzheimer and Lewy Body Dementias: Expanding Views. Kanel P, Bedard MA, Aghourian M, Rosa-Neto P, Soucy JP, Albin RL, Bohnen NI. Curr Neurol Neurosci Rep. 2021 Sep 20;21(10):52. doi: 10.1007/s11910-021-01140-z. PMID: 34545424; PMCID: PMC9020458.
- Differential cholinergic systems' changes in progressive supranuclear palsy versus Parkinson's disease: an exploratory analysis. Kanel P, Spears CC, Roytman S, Koeppe RA, Frey KA, Scott PJH, Albin RL, Bohnen NI. Journal of neural transmission (Vienna, Austria : 1996). 2022 December;129(12):1469-1479. PubMed PMID: 36222971; PubMed Central PMCID: PMC10017092; DOI: 10.1007/s00702-022-02547-9.
- Automated Synthesis of 18F-BCPP-EF {2-tert-Butyl-4-Chloro-5-{6-[2-(2[18F]fluoroethoxy)-Ethoxy]-Pyridin-3-ylmethoxy}-2H-Pyridazin-3-One for Imaging of Mitochondrial Complex 1 in Parkinson's Disease. Kaur T, Brooks AF, Liddell KM, Henderson BD, Hockley BG, Bohnen NI, Albin RL, Scott PJH. Front Chem. 2022 Mar 30;10:878835. doi: 10.3389/fchem.2022.878835. PMID: 35433631; PMCID: PMC9005973.
- Imaging of sleep disorders in pre-Parkinsonian syndromes. Ota Y, Kanel P, Bohnen N. Curr Opin Neurol. 2022 Aug 1;35(4):443-452. doi: 10.1097/WCO.0000000000001084. Epub 2022 Jul 5. PMID: 35788559; PMCID: PMC9308698.
- Composite measures of motor performance and self-efficacy are better determinants of postural instability and gait difficulties than individual clinical measures in Parkinson's disease. Pongmala C, Roytman S, van Emde Boas M, Dickinson O, Kanel P, Bohnen NI. Parkinsonism & related disorders. 2023 February;107:105251. PubMed PMID: 36566525; PubMed Central PMCID: PMC10028594; DOI: 10.1016/j.parkreldis.2022.105251.
- Identification of cholinergic centro-cingulate topography as main contributor to cognitive functioning in Parkinson's disease: Results from a data-driven approach. van der Zee S, Kanel P, Müller MLTM, van Laar T, Bohnen NI. Front Aging Neurosci. 2022 Oct 20;14:1006567. doi: 10.3389/fnagi.2022.1006567. PMID: 36337707; PMCID: PMC9631831.
Altered Cholinergic Innervation in De Novo Parkinson's Disease with and without Cognitive Impairment. van der Zee S, Kanel P, Gerritsen MJJ, Boertien JM, Slomp AC, Müller MLTM, Bohnen NI, Spikman JM, van Laar T. Mov Disord. 2022 Jan 17. doi: 10.1002/mds.28913. PMID: 35037719; PMCID: PMC9306739.
Automated Synthesis of 18F-BCPP-EF {2-tert-Butyl-4-chloro-5-{6-[2-(2[18F]fluoroethoxy)-ethoxy]-pyridin-3-ylmethoxy}-2H-pyridazin-3-one for Imaging of Mitochondrial Complex 1 in Parkinson’s Disease. Kaur T, Brooks AF, Liddell KM, Henderson BD, Bohnen NI, Albin RL, Scott PJH. Front Chem. 2022 Mar 30;10:878835. doi: 10.3389/fchem.2022.878835. PMID: 35433631; PMCID: PMC9005973.
Cholinergic systems, attentional-motor integration, and cognitive control in Parkinson's disease. Albin RL, van der Zee S, van Laar T, Sarter M, Lustig C, Muller MLTM, Bohnen NI Prog Brain Res. 2022;269(1):345-371. doi: 10.1016/bs.pbr.2022.01.011. Epub 2022 Feb 4. PMID: 35248201. PMCID: PMC8957710
Cerebral topography of vesicular cholinergic transporter changes in neurologically intact adults: A [18F]FEOBV PET study. Kanel P, van der Zee S, Sanchez-Catasus CA, Koeppe RA, Scott PJH, van Laar T, Albin RL, Bohnen NI. Aging Brain 2 (2022) 100039. https://doi.org/10.1016/j.nbas.2022.100039
Cholinergic brain network deficits associated with vestibular sensory conflict deficits in Parkinson's disease: correlation with postural and gait deficits. Bohnen NI, Kanel P, Roytman S, Scott PJH, Koeppe RA, Albin RL, Kerber KA, Müller MLTM. Journal of neural transmission (Vienna, Austria : 1996). 2022 August;129(8):1001-1009. PubMed PMID: 35753016; PubMed Central PMCID: PMC9308723; DOI: 10.1007/s00702-022-02523-3.
Cholinergic system changes in Parkinson's disease: emerging therapeutic approaches. Bohnen NI, Yarnall AJ, Weil RS, Moro E, Moehle MS, Borghammer P, Bedard MA, Albin RL. Lancet Neurol. 2022 Apr;21(4):381-392. doi: 10.1016/S1474-4422(21)00377-X. Epub 2022 Feb 4. PMID: 35131038; PMCID: PMC8985079.
Decreased vestibular efficacy contributes to abnormal balance in Parkinson's disease. Bohnen NI, Roytman S, Griggs A, David SM, Beaulieu ML, Müller MLTM. Journal of the neurological sciences. 2022 September 15;440:120357. PubMed PMID: 35932698; PubMed Central PMCID: PMC9444904; DOI: 10.1016/j.jns.2022.120357.
Differential cholinergic systems' changes in progressive supranuclear palsy versus Parkinson's disease: an exploratory analysis. Kanel P, Spears CC, Roytman S, Koeppe RA, Frey KA, Scott PJH, Albin RL, Bohnen NI. Journal of neural transmission (Vienna, Austria : 1996). 2022 December;129(12):1469-1479. PubMed PMID: 36222971; PubMed Central PMCID: PMC10017092; DOI: 10.1007/s00702-022-02547-9.
Discussion of Research Priorities for Gait Disorders in Parkinson's Disease. Bohnen NI, Costa RM, Dauer WT, Factor SA, Giladi N, Hallett M, Lewis SJG, Nieuwboer A, Nutt JG, Takakusaki K, Kang UJ, Przedborski S, Papa SM; MDS-Scientific Issues Committee. Mov Disord. 2022 Feb;37(2):253-263. PMID 34939221
Dopamine neuron stimulation induces context-dependent abnormal involuntary movements in healthy rats. Hunter J, Bova A, Stevens A, Leventhal DK. 2022 Feb 22;25(3):103974. doi: 10.1016/j.isci.2022.103974. PMID: 35281727; PMCID: PMC8914546.
Heterogeneous digital biomarker integration out-performs patient self-reports in predicting Parkinson's disease. Deng K, Li Y, Zhang H, Wang J, Albin RL, Guan Y. Commun Biol. 2022 Jan 17;5(1):58. doi: 10.1038/s42003-022-03002-x. PMID: 35039601; PMCID: PMC8763910.
Identification of cholinergic centro-cingulate topography as main contributor to cognitive functioning in Parkinson's disease: Results from a data-driven approach. van der Zee S, Kanel P, Müller MLTM, van Laar T, Bohnen NI. Front Aging Neurosci. 2022 Oct 20;14:1006567. doi: 10.3389/fnagi.2022.1006567. PMID: 36337707; PMCID: PMC9631831.
Imaging of sleep disorders in pre-Parkinsonian syndromes. Ota Y, Kanel P, Bohnen N. Curr Opin Neurol. 2022 Aug 1;35(4):443-452. doi: 10.1097/WCO.0000000000001084. Epub 2022 Jul 5. PMID: 35788559; PMCID: PMC9308698.
No Dopamine Agonist Modulation of Brain [18F]FEOBV Binding in Parkinson's Disease. Albin RL, Kanel P, van Laar T, van der Zee S, Roytman S, Koeppe RA, Scott PJH, Bohnen NI. Mol Pharm. 2022 Apr 4;19(4):1176-1182. doi: 10.1021/acs.molpharmaceut.1c00961. Epub 2022 Mar 15. PMID: 35289620; PMCID: PMC8983523.
Progression from Normal to MCI and from MCI to AD using Clinical Variables in the National Alzheimer’s Coordinating Center Uniform Data Set Version 3: Application of Machine Learning Models and a Probability Calculator. Pang Y, Albin RL, Kukull W, Sano M, Shen C, Zhou J, and Dodge HH. Forthcoming in the Journal of Prevention of Alzheimer's Disease
Progression of regional cortical cholinergic denervation in Parkinson's disease. Bohnen NI, Roytman S, Kanel P, Müller MLTM, Scott PJH, Frey KA, Albin RL, Koeppe RA. Brain Commun. 2022 Dec 2;4(6):fcac320. doi: 10.1093/braincomms/fcac320. PMID: 36569603; PMCID: PMC9772878.
Running speed and REM sleep control two distinct modes of rapid interhemispheric communication. Ghosh M, Yang FC, Rice SP, Hetrick V, Gonzalez AL, Siu D, Brennan EKW, John TT, Ahrens AM, Ahmed OJ. Cell reports. 2022 July 5;40(1):111028. PubMed PMID: 35793619; PubMed Central PMCID: PMC9291430; DOI: 10.1016/j.celrep.2022.111028.
Serotonin Transporter Imaging in Multiple System Atrophy and Parkinson's Disease. Chou KL, Dayalu P, Koeppe RA, Gilman S, Spears CC, Albin RL, Kotagal V. Mov Disord. 2022 Nov;37(11):2301-2307. doi: 10.1002/mds.29220. Epub 2022 Sep 14. PMID: 36102173; PMCID: PMC9669145.
Striatal Acetylcholine-Dopamine Imbalance in Parkinson Disease: In Vivo Neuroimaging Study with Dual-Tracer PET and Dopaminergic PET-Informed Correlational Tractography. Sanchez-Catasus CA, Bohnen NI, D'Cruz N, Müller MLTM. J Nucl Med. 2022 Mar;63(3):438-445. doi: 10.2967/jnumed.121.261939. Epub 2021 Jul 16. PMID: 34272323. PMCID: PMC8978203.
Vestibular Sensory Conflict During Postural Control, Freezing of Gait, and Falls in Parkinson's Disease. Bohnen NI, Kanel P, van Emde Boas M, Roytman S, Kerber KA. Mov Disord. 2022 Nov;37(11):2257-2262. doi: 10.1002/mds.29189. Epub 2022 Aug 15. PMID: 36373942; PMCID: PMC9673158.
Molecular Imaging of the Cholinergic System in Alzheimer and Lewy Body Dementias: Expanding Views. Kanel P, Bedard MA, Aghourian M, Rosa-Neto P, Soucy JP, Albin RL, Bohnen NI. Curr Neurol Neurosci Rep. 2021 Sep 20;21(10): doi: 10.1007/s11910-021-01140-z. PMID: 34545424.
Theta-gamma coupling emerges from spatially heterogeneous cholinergic neuromodulation. Yang Y, Gritton H, Sarter M, Aton SJ, Booth V, Zochowski M. PLoS Comput Biol 2021 Jul;17(7):e1009235. PMCID: PMC8357148.
Reduction of falls in a rat model of PD falls by the M1 PAM TAK-071. Kucinski A, Sarter M. Psychopharmacology (Berl) 2021 Jul;238(7):1953-1964. PMCID: PMC7969347
α4β2*Nicotinic Cholinergic Receptor Target Engagement in Parkinson Disease Gait-Balance Disorders. Albin RL, Müller MLTM, Bohnen NI, Spino C, Sarter M, Koeppe RA, Szpara A, Kim K, Lustig C, Dauer WT. Ann Neurol. 2021 Jul;90(1):130-142. doi: 10.1002/ana.26102. Epub 2021 May 26. PMID: 33977560. PMCID: PMC9013471
Repeat RNA Toxicity Drives Ribosomal RNA Processing Defects in SCA2. Skariah G, Albin RL. Mov Disord. 2021 Nov;36(11):2464-2467. doi: 10.1002/mds.28795. PMID: 34783387; PMCID: PMC8604384.
Evolution of Gross Forelimb and Fine Digit Kinematics during Skilled Reaching Acquisition in Rats. Bova A, Ferris K, Leventhal DK. 2021 Oct 27;8(5):ENEURO.0153-21.2021. doi: 10.1523/ENEURO.0153-21.2021. PMID: 34625461; PMCID: PMC8555885.
Regional cerebral cholinergic nerve terminal integrity and cardinal motor features in Parkinson's disease. Bohnen NI, Kanel P, Koeppe RA, Sanchez-Catasus CA, Frey KA, Scott P, Constantine GM, Albin RL, Müller MLTM. Brain Commun. 2021 May 22;3(2):fcab109. doi: 10.1093/braincomms/fcab109. PMID: 34704022; PMCID: PMC8196256.
Cholinergic Denervation Patterns Across Cognitive Domains in Parkinson's Disease. van der Zee S, Müller MLTM, Kanel P, van Laar T, Bohnen NI. Mov Disord. 2021 Mar;36(3):642-650. doi: 10.1002/mds.28360. Epub 2020 Nov 2. PMID: 33137238; PMCID: PMC7988481.
Make a Left Turn: Cortico-Striatal Circuitry Mediating the Attentional Control of Complex Movements. Sarter M, Avila C, Kucinski A, Donovan E. Mov Disord. 2021 Mar;36(3):535-546. doi: 10.1002/mds.28532. Epub 2021 Feb 22. PMID: 33615556; PMCID: PMC8938956.
Precisely timed dopamine signals establish distinct kinematic representations of skilled movements. Bova A, Gaidica M, Hurst A, Iwai Y, Hunter J, Leventhal DK. Elife. 2020 Nov 27;9:e61591. doi: 10.7554/eLife.61591. PMID: 33245045; PMCID: PMC7861618.
New Developments in Cholinergic Imaging in Alzheimer and Lewy Body Disorders. Craig CE, Ray NJ, Müller MLTM, Bohnen NI. Curr Behav Neurosci Rep. 2020 Dec;7(4):278-286. doi: 10.1007/s40473-020-00221-6. Epub 2020 Oct 9. PMID: 33777646; PMCID: PMC7992054.
Phasic cholinergic signaling promotes emergence of local gamma rhythms in excitatory-inhibitory networks. Lu Y, Sarter M, Zochowski M, Booth V. Eur J Neurosci 2020;52(6):3545-3560. PMCID: PMC8054224
Dopaminergic Nigrostriatal Connectivity in Early Parkinson Disease: In Vivo Neuroimaging Study of 11C-DTBZ PET Combined with Correlational Tractography. Sanchez-Catasus CA, Bohnen NI, Yeh FC, D'Cruz N, Kanel P, Müller MLTM. J Nucl Med. 2021 Apr;62(4):545-552. doi: 10.2967/jnumed.120.248500. Epub 2020 Aug 28. PMID: 32859707. PMCID: PMC8973250.
Molecular Imaging of the Cholinergic System in Alzheimer and Lewy Body Dementias: Expanding Views. Kanel P, Bedard MA, Aghourian M, Rosa-Neto P, Soucy JP, Albin RL, Bohnen NI. Curr Neurol Neurosci Rep. 2021 Sep 20;21(10):52. doi: 10.1007/s11910-021-01140-z. PMID: 34545424; PMCID: PMC9020458.
- Aging, Vestibular Function, and Balance: Proceedings of a National Institute on Aging/National Institute on Deafness and Other Communication Disorders Workshop. Agrawal Y, Merfeld DM, Horak FB, Redfern MS, Manor B, Westlake KP, Holstein GR, Smith PF, Bhatt T, Bohnen NI, Lipsitz LA.. J Gerontol A Biol Sci Med Sci. 2020 Nov 13;75(12):2471-2480. doi: 10.1093/gerona/glaa097. PMID: 32617555; PMCID: PMC7662183.
- Cholinergic Denervation Patterns Across Cognitive Domains in Parkinson's Disease. van der Zee S, Müller MLTM, Kanel P, van Laar T, Bohnen NI. Mov Disord. 2020 Nov 2. doi: 10.1002/mds.28360. Epub ahead of print. PMID: 33137238.
- Body-first versus brain-first biological subtyping of Parkinson's disease. Bohnen NI, Postuma RB. Brain. 2020 Oct 1;143(10):2871-2873. doi: 10.1093/brain/awaa293. PMID: 33103732. PMC Journal in process
- A failed future. Albin RL, Kordower JH. Mov Disord. 2020 Aug;35(8):1299-1301. doi: 10.1002/mds.28130. PMID: 32780511.
- Resting state connectivity within the basal ganglia and gait speed in older adults with cerebral small vessel disease and locomotor risk factors. Karim HT, Rosso A, Aizenstein HJ, Bohnen NI, Studenski S, Rosano C. Neuroimage Clin. 2020 Aug 28;28:102401. doi: 10.1016/j.nicl.2020.102401. Epub ahead of print. PMID: 32932053; PMCID: PMC7495101.
- Vulnerabilities of Aging and Biological Effects of Physical Activity Provide New Clues for Interventions in Parkinson's Disease. Bohnen NI. J Gerontol A Biol Sci Med Sci. 2020 Mar 9;75(4):687-689. doi: 10.1093/gerona/glaa026. PMID: 32150615. PMC Journal in process
- Compressive Big Data Analytics: An ensemble meta-algorithm for high-dimensional multisource datasets. Marino S, Zhao Y, Zhou N, Zhou Y, Toga AW, Zhao L, Jian Y, Yang Y, Chen Y, Wu Q, Wild J, Cummings B, Dinov ID. PLoS One. 2020 Aug 28;15(8):e0228520. doi: 10.1371/journal.pone.0228520. PMID: 32857775; PMCID: PMC7455041.
- Development and validation of the automated imaging differentiation in Parkinsonism (AID-P): A multi-site machine learning study. Archer DB, Bricker JT, Chu WT, Burciu RG, Mccracken JL, Lai S, Coombes SA, Fang R, Barmpoutis A, Corcos DM, Kurani AS, Mitchell T, Black ML, Herschel E, Simuni T, Parrish TB, Comella C, Xie T, Seppi K, Bohnen NI, Müller MLTM, Albin RL, Krismer F, Du G, Lewis MM, Huang X, Li H, Pasternak O, McFarland NR, Okun MS, Vaillancourt DE. Lancet Digit Health. 2019 Sep;1(5):e222-e231. doi: 10.1016/s2589-7500(19)30105-0. Epub 2019 Aug 27. PMID: 32259098; PMCID: PMC7111208.
- Current and projected future economic burden of Parkinson's disease in the U.S. Yang W, Hamilton JL, Kopil C, Beck JC, Tanner CM, Albin RL, Ray Dorsey E, Dahodwala N, Cintina I, Hogan P, Thompson T. NPJ Parkinsons Dis. 2020 Jul 9;6:15. doi: 10.1038/s41531-020-0117-1. PMID: 32665974; PMCID: PMC7347582.
- Seizure occurrence and related mortality in dementia with Lewy bodies. Marawar R, Wakim N, Albin RL, Dodge H. Epilepsy Behav. 2020 Jul 18;111:107311. doi: 10.1016/j.yebeh.2020.107311. Epub ahead of print. PMID: 32693380. PMCID: PMC7541707
- Deep learning identifies digital biomarkers for self-reported Parkinson's disease. Zhang H, Deng K, Li H, Albin RL, Guan Y. Patterns (N Y). 2020 Jun 12;1(3):100042. doi: 10.1016/j.patter.2020.100042. Epub 2020 May 28. PMID: 32699844; PMCID: PMC7375444.
- Topography of cholinergic changes in dementia with Lewy bodies and key neural network hubs. Kanel P, Müller MLTM, van der Zee S, Sanchez-Catasus CA, Koeppe RA, Frey KA, Bohnen NI. J Neuropsychiatry Clin Neurosci. 2020 Jun 5:appineuropsych19070165. doi: 10.1176/appi.neuropsych.19070165. Epub ahead of print. PMID: 32498602.
- Phasic cholinergic signaling promotes emergence of local gamma rhythms in excitatory-inhibitory networks. Lu Y, Sarter M, Zochowski M, Booth V. [published online ahead of print, 2020 Apr 15]. Eur J Neurosci. 2020;10.1111/ejn.14744. doi:10.1111/ejn.14744
- Forebrain cholinergic signaling: Wired and phasic, not tonic, and causing behavior. Sarter M, Lustig C. J Neurosci. 2020 Jan 22;40(4):712-719. doi: 10.1523/JNEUROSCI.1305-19.2019. PMID: 31969489 PMCID: PMC6975286
- Diffusion magnetic resonance imaging-derived free water detects neurodegenerative pattern induced by interferon-γ. Febo M, Perez PD, Ceballos-Diaz C, et al. Brain Struct Funct. 2020;225(1):427-439. doi:10.1007/s00429-019-02017-1. PMID: 31894407 PMCID: PMC7003714
- Correction to: Co-treatment with rivastigmine and idalopirdine reduces the propensity for falls in a rat model of falls in Parkinson's disease. Cherian AK, Kucinski A, Wu R, de Jong IEM, Sarter M. Psychopharmacology (Berl). 2020;237(1):289. doi:10.1007/s00213-019-05375-0. PMID: 31650233
- HDQLIFE and neuro-QoL physical function measures: Responsiveness in persons with huntington's disease. Carlozzi NE, Boileau NR, Chou KL, Ready RE, Cella D, McCormack MK, Miner JA, Dayalu P. Mov Disord. 2020;35(2):326-336. doi:10.1002/mds.27908. PMID: 31724237 PMCID: PMC7041888.
- Complex movement control in a rat model of Parkinsonian falls: bidirectional control by striatal cholinergic interneurons. Sarter M, Avila C, Kucinski A. J Neurosci. 2020 Jun 18:JN-RM-0220-20. doi: 10.1523/JNEUROSCI.0220-20.2020. Epub ahead of print. PMID: 32554512. PMCID: PMC7392507.
- Apathy rating scores and β-amyloidopathy in patients with Parkinson disease at risk for cognitive decline. Zhou Z, Müller MLTM, Kanel P, Chua J, Kotagal V, Kaufer DI, Albin RL, Frey KA, Bohnen NI. Neurology. 2020 Jan 28;94(4):e376-e383. doi: 10.1212/WNL.0000000000008683. Epub 2019 Nov 15. PMID: 3173256 PMCID: PMC7079689
- Increased striatal dopamine in carriers of GBA mutations: compensation or epiphenomenon? Bohnen NI, Albin RL. Lancet Neurol. 2020 Jan;19(1):27-29. doi: 10.1016/S1474-4422(19)30355-2. Epub 2019 Oct 31. No abstract available. PMID: 31678033. PMCID: PMC6924570
- Freezing of gait: understanding the complexity of an enigmatic phenomenon. Weiss D, Schoellmann A, Fox MD, Bohnen NI, Factor SA, Nieuwboer A, Hallett M, Lewis SJG. Brain. 2020 Jan 1;143(1):14-30. doi: 10.1093/brain/awz314. PMID: 31647540 PMCID: PMC6938035
- Precisely timed dopamine signals establish distinct kinematic representations of skilled movements. Bova A, Gaidica M, Hurst A, Iwai Y, Hunter J, Leventhal DK. Elife. 2020 Nov 27;9:e61591. doi: 10.7554/eLife.61591. PMID: 33245045; PMCID: PMC7861618.
- New Developments in Cholinergic Imaging in Alzheimer and Lewy Body Disorders. Craig CE, Ray NJ, Müller MLTM, Bohnen NI. Curr Behav Neurosci Rep. 2020 Dec;7(4):278-286. doi: 10.1007/s40473-020-00221-6. Epub 2020 Oct 9. PMID: 33777646; PMCID: PMC7992054.
- Tourette Syndrome as a Disorder of the Social Decision Making Network. Albin RL. Front Psychiatry. 2019 Oct 8;10:742. doi: 10.3389/fpsyt.2019.00742. PMID: 31649568; PMCID: PMC6792345.
- Treatment options for postural instability and gait difficulties in Parkinson's disease. Müller MLTM, Marusic U, van Emde Boas M, Weiss D, Bohnen NI. Expert Rev Neurother. 2019 Dec;19(12):1229-1251. doi: 10.1080/14737175.2019.1656067. Epub 2019 Aug 30. PMID: 31418599
- HDDA: DataSifter: Statistical obfuscation of electronic health records and other sensitive datasets. Marino S, Zhou N, Zhao Yi, Wang L, Wu Q, Dinov ID. Journal of Statistical Computation and Simulation, Vol 89:2, 249-271. 2019.doi: 10.1080/00949655.2018.1545228. PMID: 30962669. PMCID: PMC6450541
- Co-treatment with rivastigmine and idalopirdine reduces the propensity for falls in a rat model of falls in Parkinson’s disease.Koshy Cherian A, Kucinski A, Wu R, de Jong IEM, Sarter M. Psychopharmacology 04 January 2019, 1-15. PMID: 30607479
- The contribution of cerebrovascular risk factors, metabolic and inflammatory changes to cognitive decline in Parkinson’s disease - preliminary observations. Veselý B, Koriťáková E, Bohnen NI, Viszlayová D, Királová S, Valkovič P, Kurča E & Rektor I (2019) J Neural Transm (Vienna).2019 Oct;126(10):1303-1312. doi: 10.1007/s00702-019-02043-7. Epub 2019 Jul 22. PMID: 31332506. PMCID: PMC6959128
- The open diffusion data derivatives, brain data upcycling via integrated publishing of derivatives and reproducible open cloud services.Avesani P, McPherson B, Hayashi S, Caiafa CF, Henschel R, Garyfallidis E, Kitchell L, Bullock D, Patterson A, Olivetti E, Sporns O, Saykin AJ, Wang L, Dinov I, Hancock D, Caron B, Qian Y, Pestilli F. Sci Data. 2019 May 23;6(1):69. doi:10.1038/s41597-019-0073-y. PubMed PMID: 31123325; PubMed Central PMCID: PMC6533280.
- Predictive big data analytics using the UK Biobank Data. Zhou Y, Zhao L, Zhou N, Zhao Y, Marino S, Wang T, Sun H, Toga AW, Dinov ID. Sci Rep. 2019 Apr 12;9(1):6012. doi: 10.1038/s41598-019-41634-y. PubMed PMID: 30979917; PubMed Central PMCID: PMC6461626.
- Fatigue in Parkinson's disease associates with lower ambulatory diastolic blood pressure. Kotagal V, Szpara A, Albin RL, Bohnen NI. J Parkinsons Dis.2019;9(3):575-581. doi: 10.3233/JPD-191579. PubMed PMID: 31156183; PubMed Central PMCID: PMC6682445.
- Polyglutamine repeats in neurodegenerative diseases. Lieberman AP, Shakkottai VG, Albin RL. Annu Rev Pathol. 2019 Jan 24;14:1-27. doi:10.1146/annurev-pathmechdis-012418-012857. Epub 2018 Aug 8. PubMed PMID:30089230; PubMed Central PMCID: PMC6387631.
- Complementary motivational roles of nigroaccumbens and nigrostriataldopaminergic pathways.Mov Disord. Albin RL. 2019 Jan;34(1):45. doi: 10.1002/mds.27504.PubMed PMID: 30653732. PubMed Central PMCID: PMCID:PMC6812511
- The cortical cholinergic system contributes to the top-down control of distraction: Evidence from patients with Parkinson's disease.Kim K, Müller, MLT, Bohnen NI, Sarter M, Lustig C. (2019). NeuroImage, 190, 107-117. PubMed Central PMCID: PMCID: PMC6008164
- Sleep disturbance as potential risk and progression factor for Parkinson's disease.Bohnen NI, Hu MTM. J Parkinsons Dis. 2019;9(3):603-614. doi: 10.3233/JPD-191627. PMID: 31227656. PubMed Central PMCID: PMC6700634
- Effects of plasma glucose levels on regional cerebral 18F-fluorodeoxyglucose uptake: Implications for dementia evaluation with brain PET imaging.Viglianti BL, Wale DJ, Ma T, Johnson TD, Bohnen NI, Wong KK, Ky C, Frey KA, Townsend DM, Rubello D, Gross MD. Biomed Pharmacother. 2019 Apr;112:108628. doi: 10.1016/j.biopha.2019.108628. Epub 2019 Feb 20. PMID: 30784923 PubMed Central PMCID: PMC6714976
- Neuroimaging biomarkers for clinical trials in atypical parkinsonian disorders: Proposal for a Neuroimaging Biomarker Utility System.van Eimeren T, Antonini A, Berg D, Bohnen N, Ceravolo R, Drzezga A, Höglinger GU, Higuchi M, Lehericy S, Lewis S, Monchi O, Nestor P, Ondrus M, Pavese N, Peralta MC, Piccini P, Pineda-Pardo JÁ, Rektorová I, Rodríguez-Oroz M, Rominger A, Seppi K, Stoessl AJ, Tessitore A, Thobois S, Kaasinen V, Wenning G, Siebner HR, Strafella AP, Rowe JB. Alzheimers Dement (Amst). 2019 Apr 2;11:301-309. doi: 10.1016/j.dadm.2019.01.011. eCollection 2019 Dec. PubMed PMID: 30984816; PubMed Central PMCID: PMC6446052.
- Cholinergic system changes of falls and freezing of gait in Parkinson disease. Bohnen NI, Kanel P, Zhou Z, Koeppe RA, Frey KA, Dauer WT, Albin RL, Müller MLTM Annals of Neurology. 2019 Feb 5. doi: 10.1002/ana.25430. [Epub ahead of print]. PMID: 30720884 PMCID: PMC6450746
- Multimodal dopaminergic and free-water imaging in Parkinson's disease.Yang J, Archer DB, Burciu RG, Müller MLTM, Roy A, Ofori E, Bohnen NI, Albin RL, Vaillancourt DE. Parkinsonism Relat Disord. 2019 May;62:10-15. doi: 10.1016/j.parkreldis.2019.01.007. Epub 2019 Jan 6. PubMed PMID: 30639168; PubMed Central PMCID: PMC6589363.
- Quantification of brain cholinergic denervation in dementia with Lewy bodies using PET imaging with [(18)F]-FEOBV.Nejad-Davarani S, Koeppe RA, Albin RL, Frey KA, Müller MLTM, Bohnen NI. Mol Psychiatry. 2019 Mar;24(3):322-327. doi: 10.1038/s41380-018-0130-5. Epub 2018 Aug 6. PubMed PMID: 30082840; PubMed Central PMCID: PMC6363916.
- Cholinergic double duty: cue detection and attentional control.Sarter M, Lustig C. Curr Opin Psychol. 2019, 29:102-107. PubMed PMID: 30711909; PMCID: PMC6609491
- Basal forebrain chemogenetic inhibition disrupts the superior complex movement control of goal-tracking rats. Kucinski A, Kim Y, Sarter M. Behavioral Neuroscience 133(1), 121-134. 2019. doi: 10.1037/bne0000290. DOI: 1037/bne0000290 PMID:30688488 PMCID: PMC6850517
- The open diffusion data derivatives, brain data upcycling via integrated publishing of derivatives and reproducible open cloud services. Avesani P, McPherson B, Hayashi S, Caiafa CF, Henschel R, Garyfallidis E, Kitchell L, Bullock D, Patterson A, Olivetti E, Sporns O, Saykin AJ, Wang L, Dinov I, Hancock D, Caron B, Qian Y, Pestilli F. Sci Data. 2019 May 23;6(1):69. doi: 10.1038/s41597-019-0073-y. PMID: 31123325; PMCID: PMC6533280.
- Model-based and model free techniques for Amyotrophic Lateral Sclerosis diagnostic prediction and patient clustering. Tang M, Gao C, Goutman SA, Kalinin A, Mukherjee B, Guan Y, Dinov ID. Neuroinformatics. 2019 Jul;17(3):407-421. doi: 10.1007/s12021-018-9406-9. PMID: 30460455; PMCID: PMC6527505.
- Machine learning techniques for personalized breast cancer risk prediction: comparison with the BCRAT and BOADICEA models. Ming C, Viassolo V, Probst-Hensch N, Chappuis PO, Dinov ID, Katapodi MC Breast Cancer Res. 2019;21(1):75. Published 2019 Jun 20. doi:10.1186/s13058-019-1158-4. PMID: 31221197. PMCID: PMC6585114850517
Mac Shine – Updating our models of the basal ganglia using advances in neuroanatomy and computational modelling
Bernardo Sabatini - Basal ganglia circuits for action selection and evaluation
Shelly Flagel - Exploiting individual differences in motivated behavior to identify the neural processes underlying neuropsychiatric disorders
Honglei Chen - Research on environmental risk factors of Parkinson’s disease – challenges and strategies
Christian Burgess - Striatal dopamine dynamics during skilled motor learning
Michael Vesia - Measuring and manipulating brain circuits for goal-directed movements with multi-focal transcranial magnetic stimulation
Prabesh Kanel - Voxel-based principal component analysis of brain PET images
Megha Ghosh - Rhythms of the retrosplenial cortex during movement and REM sleep
Sam Crowley, Alex Johnson, James Brissenden - The basal forebrain, acetylcholine, and cognition in Parkinson's disease; Electrophysiological signatures of a basal forebrain-retrosplenial system; Restoration of vigor-motivation coupling in Parkinson's disease requires both the short and long duration response to L-Dopa
Per Borghammer - Brain-first vs body-first Parkinson’s disease- the idea, validation studies, and implications
Hong-Yuan Chu - Cortical cellular and synaptic dysfunction in Parkinsonism
Simon Lewis - Parkinson’s and Phrenology for the 21st Century
Yoland Smith - Pathophysiology of Thalamocortical and Corticofugal Systems in Parkinsonism
Benjamin Hampstead - Non-invasive brain stimulation in Parkinson’s and Lewy Body dementia
Kumar Narayanan - Cognition and 4 Hz Rhythms in Parkinson’s Disease
Cassandra Avila - The role of corticostriatal interactions in complex movement
This mentored research training program in basic and clinical neuroscience has been funded continuously by the NIH/NINDS since 1982 and has served more than 115 trainees. The program involves the Departments of Neurology, Pediatrics, Neurosurgery, Internal Medicine and Anesthesia at the University of Michigan (U-M) and the Ann Arbor VA Medical Centers. Trainees have access to 35,000 square feet of laboratory space for basic research; more than 45 faculty members, most of whom are both clinical neurologists and basic or clinician scientists, serve as preceptors. Learn more.
The majority of neuroscience majors plan to further their education by attending professional schools in medicine, veterinary medicine, dentistry, and pharmacy. Others go on to graduate school programs in a variety of fields (neuroscience, psychology, biology, microbiology, cellular & molecular biology, and education, to name a few). Still others obtain jobs in industry, sales, education, or with the government. There are many options! Learn more.
The majority of neuroscience majors plan to further their education by attending professional schools in medicine, veterinary medicine, dentistry, and pharmacy. Others go on to graduate school programs in a variety of fields (neuroscience, psychology, biology, microbiology, cellular & molecular biology, and education, to name a few). Still others obtain jobs in industry, sales, education, or with the government. There are many options! Learn more.
The Michigan Institute for Data Science (MIDAS) is the focal point for the new multidisciplinary area of data science at the University of Michigan. This area covers a wide spectrum of scientific pursuits (development of concepts, methods, and technology) for data collection, management, analysis, and interpretation as well as their innovative use to address important problems in science, engineering, business, and other areas.
The purpose of this initiative is to co-create a bold vision and design a partnership model between the University of Michigan School of Nursing (UMSN) and University of Michigan Health System Nursing Services to advance and unite clinical education, practice and scholarship. Learn more.
The Charter of the Analytics Core is to provide doctoral students with rigorous analytics training and learning experience via a robust series of analytic courses offered by UMSN faculty. The 4-course 4-credit hours course series, Scientific Methods for Health Sciences, aims to reduce the variation of analytic skills, scientific knowledge and quantitative abilities of the health sciences graduate students. The unified objective of these courses is to prepare exclusive cadres of nursing and healthcare scientists capable of leading scientific teams in the conception of research proposals with strong methodological designs and analytic approaches to address innovative study aims. Learn more.
The Parkinson’s Progression Markers Initiative (PPMI) is a landmark observational clinical study to comprehensively evaluate cohorts of significant interest using advanced imaging, biologic sampling and clinical and behavioral assessments to identify biomarkers of Parkinson’s disease progression. Learn more.
The brain makes us who we are, largely defining our uniqueness as individuals and as a species. For this reason diseases that attack the nervous system – whether suddenly as in stroke, insidiously as in Alzheimer’s disease, or with recurrent attacks as may occur in multiple sclerosis – strike at the core of our being. Imagine being unable to speak, being unable to move your limbs at will, or experiencing difficulty recognizing common surroundings or close relatives.
In the Department of Neurology at the University of Michigan, a dedicated team of academic neurologists pursues the three-part academic mission of clinical care, research and education. Highly skilled physicians with subspecialty expertise in each of the domains of adult neurology provide care to patients with diseases of the nervous system in both hospital and outpatient settings. Many of these physicians direct laboratory or clinical research programs to advance our understanding of neurological disease, and to develop and test new therapies for these diseases.
In the United States, an estimated 42 million people suffer from some form of movement disorder such as Parkinson’s disease, essential tremor, dystonia, chorea, hemifacial spasm or restless legs syndrome. The University of Michigan Movement Disorder Program and its faculty have international reputations for multidisciplinary patient care, research and clinical education directed towards the understanding and treatment of these diseases.
Our program combines expertise in neurology, neurosurgery, neuroradiology, neuropsychology, speech pathology, and physical medicine and rehabilitation to provide a broad-based approach to treating and managing patients with movement disorders.
We make life better for people with Parkinson’s through expert care and research. Everything we do helps people actively enjoy life with their friends, families, children and grandchildren until there is a tomorrow without Parkinson’s. Learn more.
The Michael J. Fox Foundation is dedicated to finding a cure for Parkinson’s disease through an aggressively funded research agenda and to ensuring the development of improved therapies for those living with Parkinson’s today. Learn more.
The mission of NINDS is to seek fundamental knowledge about the brain and nervous system and to use that knowledge to reduce the burden of neurological disease. To support this mission, NINDS:
- Supports and performs basic, translational, and clinical neuroscience research through grants-in-aid, contracts, scientific meetings, and through research in its own laboratories, and clinics.
- Funds and conducts research training and career development programs to increase basic, translational and clinical neuroscience expertise and ensure a vibrant, talented, and diverse work force.
- Promotes the timely dissemination of scientific discoveries and their implications for neurological health to the public, health professionals, researchers, and policy-makers.
The Scientific Methods for Health Sciences (SMHS) EBook is designed to support a 4-course training curriculum emphasizing the fundamentals, applications and practice of scientific methods specifically for graduate students in the health sciences. Learn more.
The National Institute of Neurological Disorders and Stroke (NINDS) and other Federal agencies and international organizations have the common mission of developing data standards for clinical research. Through the efforts of subject-specific working groups, topic-driven data elements have been created. Learn more.
The Statistics Online Computational Resource (SOCR) designs, validates and freely disseminates knowledge. Specifically, SOCR provides portable online aids for probability, statistics and health science education, promotes technology-enhanced instruction, and supports efficient statistical computing. The SOCR platform includes a repository of interactive applets, computational web apps, graphing tools, instructional resources, learning materials, and curricular components. Learn more.