
Soleimanpour Lab Overview
Our lab is primarily focused on the control of pancreatic islet beta cell function in diabetes by all aspects of the mitochondrial life cycle.
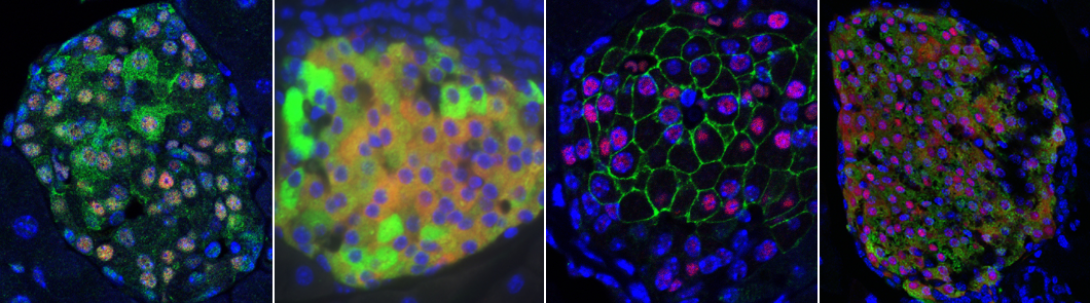
All forms of diabetes share the common etiology of insufficient insulin release from pancreatic islet beta cells to meet peripheral insulin demand. Beta cells require mitochondrial function in order to maintain proper glucose stimulated insulin release.
Our lab is primarily focused on the control of pancreatic islet beta cell function in diabetes by all aspects of the mitochondrial life cycle: biogenesis, dynamics, and mitophagy.
We utilize genetic mouse models, in vivo mitochondrial biosensors, high resolution imaging, flow cytometry, high throughput sequencing technologies, as well as human islet model systems and ES-derived beta cells to conduct our studies. All projects have a translational link, and we strive to use human islets from donors with diabetes whenever possible.
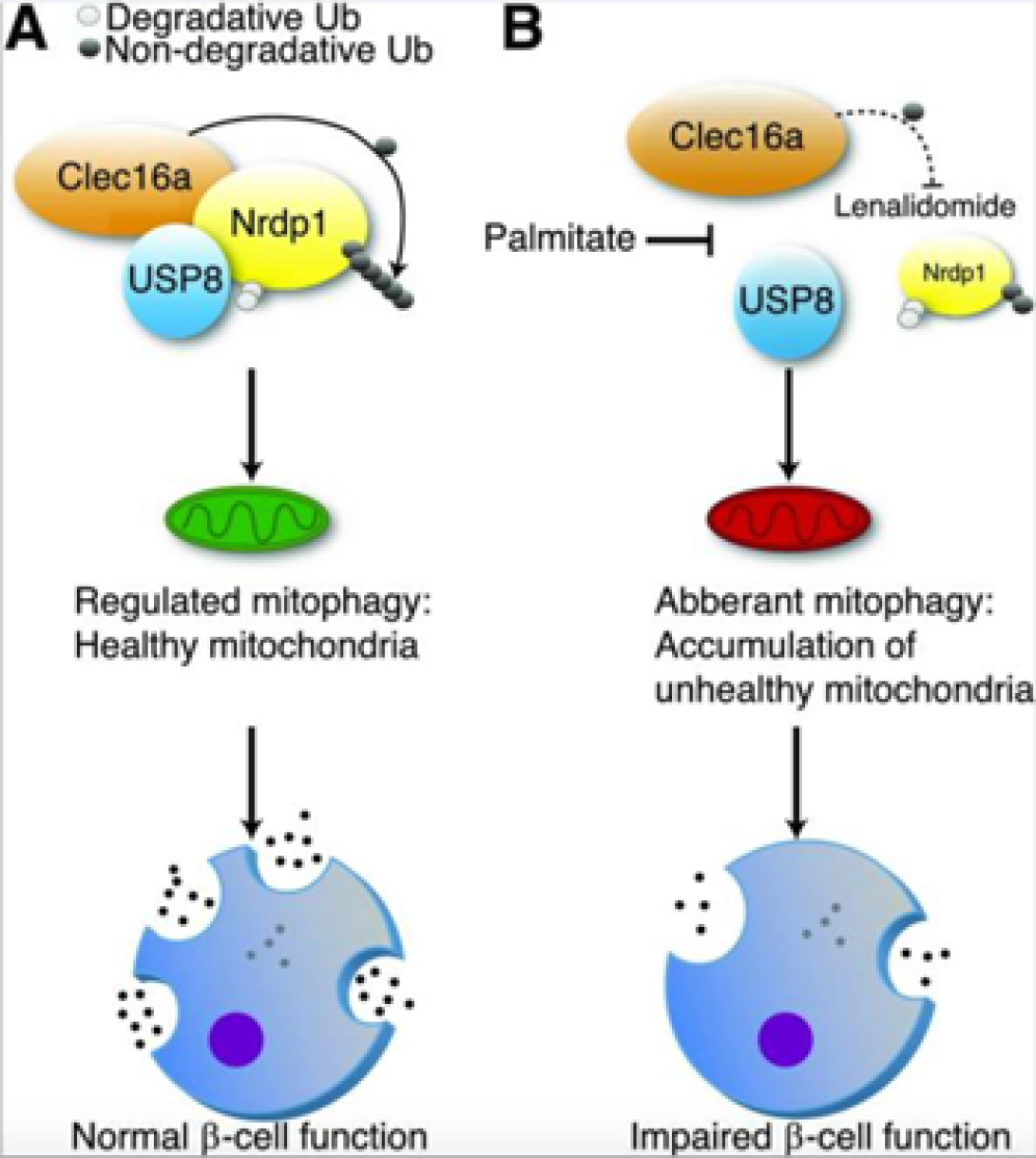
Mitophagy is a cellular quality-control pathway essential for elimination of unhealthy mitochondria. We discovered that ubiquitination is essential for the assembly of a mitophagy regulatory complex, comprised of the E3 ligase Nrdp1, the deubiquitinase enzyme USP8, and Clec16a, a mediator of β-cell mitophagy and diabetes gene. Inhibition of the Clec16a pathway by the chemotherapeutic lenalidomide, a selective ubiquitin ligase inhibitor associated with new-onset diabetes, impairs β-cell mitophagy, oxygen consumption, and insulin secretion. Indeed, patients treated with lenalidomide develop compromised β-cell function. Thus, the Clec16a-Nrdp1-USP8 complex relies on ubiquitin signals to promote mitophagy and maintain mitochondrial quality control necessary for optimal β-cell function.
Dr. Scott Soleimanpour discusses embryonic stem cell research on NBC Nightly News in March 2009.